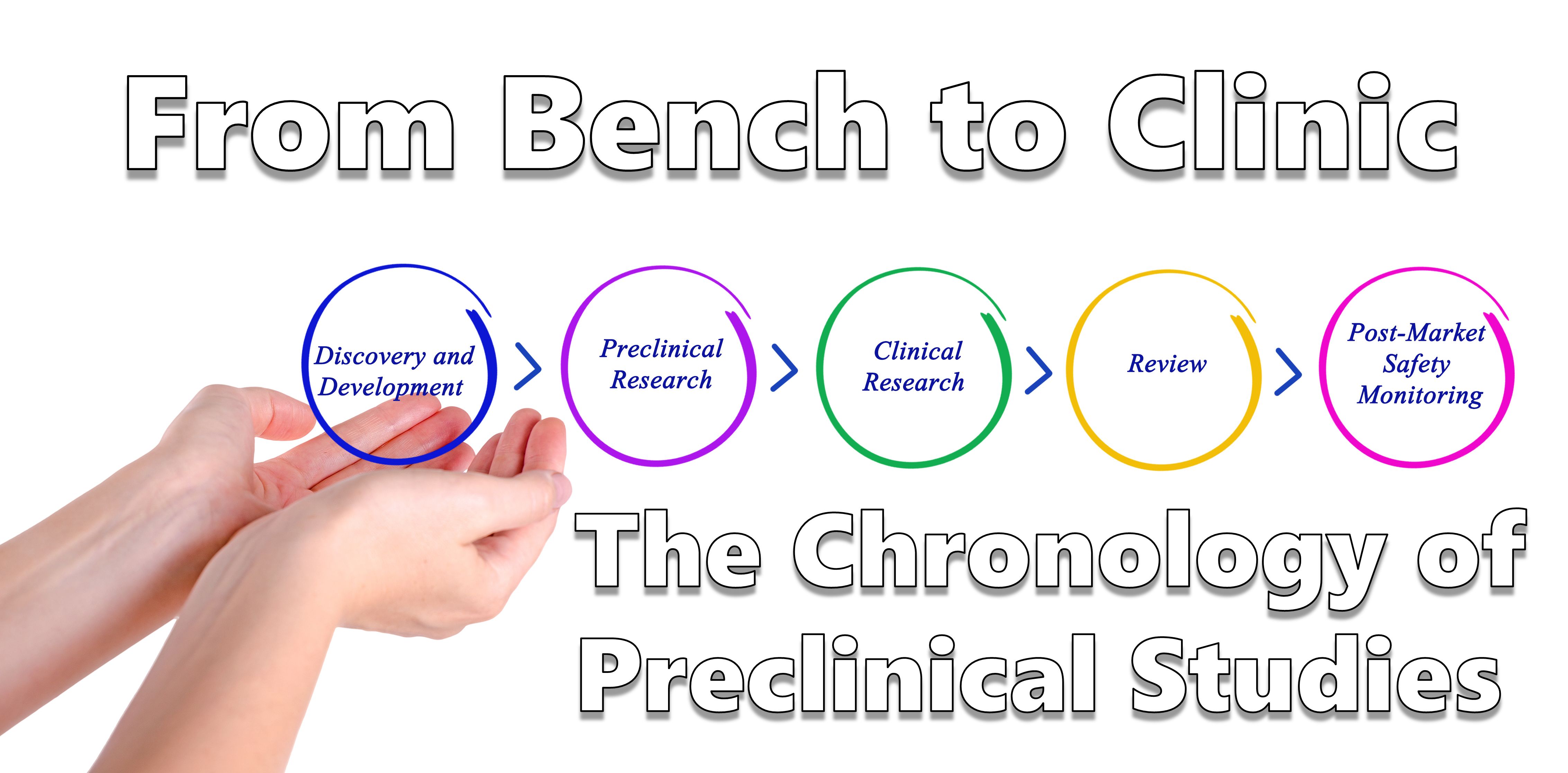
The drug development process has many phases that can take from 12 to 15 years and cost upwards of 1 billion dollars. It is critical to have a well developed roadmap and plan in mind from the earliest stages of development to minimize the amount of resources wasted through the process.
Starting the process with the end in mind is key to a smooth and efficient development.
In this newsletter, we will take a broad overview of the common tests performed during the drug development process from bench to clinic. We will briefly discuss the process of discovery and lead candidate selection and then we will describe some of the common preclinical safety tests required to submit the documentation for regulatory approval for clinical trials. Finally we will present ITRs role in this process.
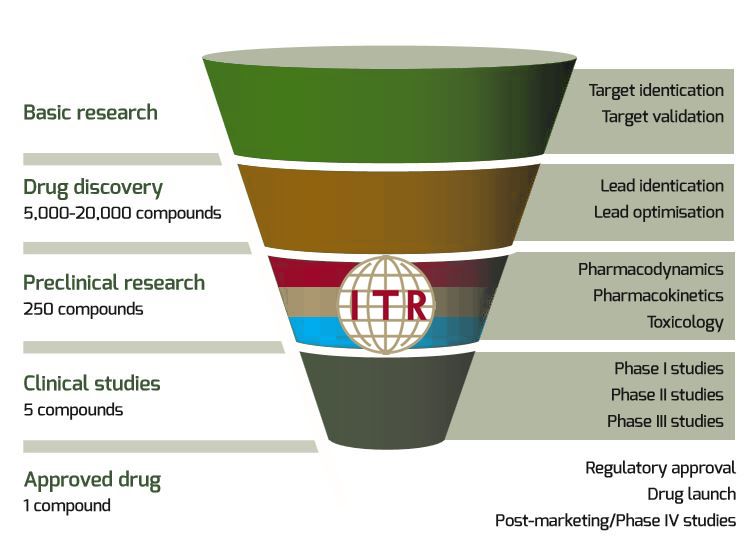
The earliest phases of drug development include drug discovery, lead candidate selection and preclinical efficacy.
The process of discovery begins with the identification of a druggable target. This process is assisted with the use of bioinformatics, phenotype screening and genetic association studies. Testing is performed to determine whether a compound alters the function of the target with a therapeutic benefit to the patient.
High-throughput screening tests the interaction between the compounds and selected cell-lines or target proteins. Large numbers of potential compounds are quickly screened until the one that binds with high affinity to the biological target is identified as potential new drug. Alternatively, low-throughput screening with preselected compounds is performed in animals, tissues or organs. Low-throughput methods are more detailed procedures, but require more time. By the time a lead candidate is identified, thousands of compounds would have been tested and rejected.
The lead compound will be selected for a number of desired criteria: specificity, potency, selectivity. The compound must have a defined method of action, it should have strong affinity for the desired target as well as selectivity for the intended target over other potential targets. Further in vivo tests are also performed to gather data on absorption, distribution, metabolism and excretion (ADME). Many drug candidates are eliminated from further development due to poor pharmacokinetic results (e.g., poor bioavailability, very short half life, etc.). As such, this early screening reduces the chances of rejection later in the development cycle.
Once the lead compound has been selected, the route and frequency of administration has been identified and efficacy has been demonstrated in vivo and/or in vitro, the next step is to ensure that the compound is safe for administration in humans. This is accomplished with a series of pre-clinical non-GLP and GLP safety studies.
Upon the completion of the primary pharmacology/efficacy studies, the next step is to determine the safety of the compound starting with the determination of the maximum tolerated dose (MTD).
The MTD is the highest dose of a drug that does not cause unacceptable side effects or overt toxicity.
MTD studies are typically single dose studies that use an escalating dose regimen in a small number of animals. The starting dose is usually equal to, or is slightly above the anticipated therapeutic/efficacious dose. Subsequent doses are increased gradually over time until mild to moderate clinical signs are observed.
The choice of animal species depends on the nature of the drug candidate. For small molecule drugs, rats and dogs are most commonly used. For large molecules, one or two species in which the test item is pharmacologically active need to be selected due to the expression of the receptor or an epitope.
Pharmacokinetic (PK) data allow researchers understand the fate of the drug in the biological system. PK studies consist of collecting serial blood samples over time, then analyzing the samples for drug concentration levels in blood, plasma or serum. PK studies are also performed in a small number of animals.
Data collected from the MTD & PK studies are then referenced to allow selection of dose levels and the dosing regimen for dose range finding studies. Range finding studies use a multi group design with a control group, a low dose, a mid dose and a high dose group and are typically 7 to 14 days long. Results obtained from these studies allow selection of dose levels for definitive repeat dose toxicity studies.
Early screening tests and tests to determine dosage information can be completed under non-GLP conditions, but taking a drug from the preclinical stage to the clinic requires a list of GLP safety studies including safety pharmacology, genetic toxicology and longer term repeat dose toxicity studies.
Safety pharmacology tests are intended to screen for undesirable effects on vital organ systems, such as the cardiovascular, respiratory, renal, gastrointestinal and central nervous systems.
Tolerance studies are performed using the intended method of administration and are not always performed with every drug. Local tolerance studies are often performed when the drug is intended to be administered topically on the skin or the eyes.
While some genetic toxicology screening tests may be performed under non-GLP conditions closer to the discovery phase, GLP genetic toxicology tests are required as part of the battery of tests for IND submissions.
Genetic toxicity testing is performed to assess for potential chromosomal damage or mutagenicity. Genetic toxicity testing consists of in vitro tests, such as the Ames test, Chromosome aberration and the in vitro micronucleus assay, as well as in vivo tests, such as the in vivo micronucleus assay.

Once the new drug candidate has undergone safety testing in single dose studies, repeat dose studies are performed under GLP conditions. These studies are intended to evaluate potential toxic effects under the intended clinical dosage regimen and method of administration and therefore, repeat dose studies should mimic the intended clinical usage as much as possible.
Repeat dose studies are performed using a relatively larger number of animals and the study design is defined by the results of the MTD, PK & range finding studies. These studies are usually performed in one rodent species as well as one non-rodent species and a typical study design will have dosing for 28 days.
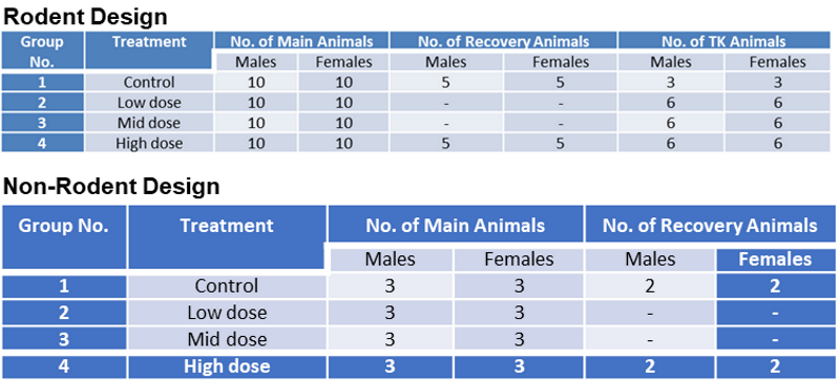
Below are examples of study designs in rodents and non-rodents. Repeat dose studies will always have a control group for statistical comparisons. A cohort of recovery animals is also typically included in definitive repeat dose toxicity studies in order to assess the delayed onset, reversibility, or persistence of toxic effects following the cessation of dosing.

ITR is a preclinical toxicology contract research organization that supports the biopharmaceutical industry by providing a wide range of non-clinical services ranging from single dose PK studies to 2-year carcinogenicity bioassays in a variety of species including mice, rats, minipigs, rabbits, dogs and monkeys. In addition to the standard battery of tests required for small molecule safety tests, we also offer a wide range of services for large molecule development in our available species.
Since 1993, ITR has been accredited by the CCAC (Canadian Council on Animal Care) and AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care). All our work is guided by standard operating procedures (SOPs) and fully compliant with the GLP (Good Laboratory Practice) including our non-GLP studies.
ITR also offers services for all methods of administration. Established as an infusion focused CRO, ITR has branched out over the last 30 years, adding world-leading inhalation expertise to our portfolio in addition to general toxicology, dermal and ocular testing.

Drug development is a long journey from the initial target identification and lead candidate selection, to the preliminary efficacy testing and safety testing. At ITR we continue to develop our safety testing expertise to suit a wider range of new drug candidates and to ensure Sponsors receive more comprehensive safety information on their drug candidates. Whether they be small molecules or large molecules, ITR helps our sponsors get their drug candidates quickly from the bench to the clinic.
