Analytical and bioanalytical sciences have a pivotal role in supporting the drug development process in both clinical and preclinical settings. From verifying accurate dosage levels to the measurement of drug concentration levels in biological matrices, analytical tools provide critical information to ensure drugs are safe and effective.
In this newsletter we will discuss a general history of analytical technologies, describe their role in the drug development process, explain the common separation and detection techniques, and present ITRs analytical and bioanalytical capabilities.
Bioanalysis has historically been used to assess the presence of drugs in biological fluids for the purpose of forensic toxicology. The development of pharmacokinetics in the 1930s propelled interest in bioanalysis for evaluating new drug candidates. Early assays lacked specificity, which presented complications in differentiating between a drug and its metabolites. Differentiation between drugs and their metabolites gained more importance when some metabolites were found to be toxic, or found to hold distinct therapeutic value.
The development of chromatographic methods to separate drugs from their metabolites began in the 1940s with paper chromatography, followed by gas chromatography (GC) in the 1950s. GC quickly became a staple technique for forensic and pharmaceutical analysis as it offered improvements in sensitivity and selectivity with more sensitive detectors becoming available, including mass spectrometers. Gas chromatography however required volatile and thermostable analytes along with complex sample preparation, which limited its utility.
The late 1960s and early 1970s saw a shift from gas chromatography towards high-pressure liquid chromatography (HPLC). This new separation method coupled with the development of ultraviolet and fluorescent detectors provided greater sensitivity to match the increasing potency of drugs under development during the 1970s and 1980s.
In preclinical and clinical settings, drugs are rarely administered in their pure form. The active pharmaceutical ingredient (API) is often delivered in a vehicle; the drug is dissolved into a solution, diluted or encased into a capsule for oral delivery, among other common methods of administration. During clinical and preclinical trials, the concentration of the API must be verified by dose formulation analysis to ensure that the correct dosage is administered. Dose formulation analysis requires separation of the API from the vehicle and other impurities so that the correct concentration of the drug can be measured.
In order to evaluate the drug’s bioavailability and fate in the biological system, data is gathered by analyzing samples of biological fluids drawn from dosed humans or animals. Accurate analysis of drug concentration in biological fluids requires the separation of drug from the biological fluid.
Today, these kinds of analysis are most often performed using gas or liquid chromatography in tandem with various detectors.
Analytical tools used today are comprised of a separation method and a detector. The selection of a tool will differ based on the chemical properties of the drug, the vehicle and the method of administration. The most frequently used separation method today is high-pressure liquid chromatography (HPLC).

HPLC uses stainless steel tubes, or columns, to house an absorbent material with a low particle size: the stationary phase. A high-pressure pump is used to pump a solvent through the stationary phase, the solvent is referred to as the mobile phase. The sample of interest is injected into the mobile phase and the movement of the mobile phase through the stationary phase in the column results in the separation of different components of the sample as they move through the columns at different speeds due to differences in affinity to the stationary phase.
At the end of each column is a detector; the type of detector used depends on the sample to be analyzed. The detector picks up each component of the sample individually based on how long they take to pass through the column, or their retention time. The computer software then generates a graphical peak to display the detection data gathered.
Each of these peaks identifies a distinct component (drug, metabolite, impurity or vehicle) and the area under the curve of each peak reveals the concentration of the corresponding component. In order to clearly identify each component, retention times must be known beforehand based on previously established standards or method development trials. HPLC is therefore most effective when analysts already know what is contained within the sample and the purpose of measurement is confirming proportions of components.
When dealing with volatile samples, gas chromatography (GC) is used instead of liquid chromatography. This is because volatile samples can be properly contained within a GC system where they cannot be contained with liquid chromatography. In GC, an inert gas is used as a mobile phase, samples are vaporized and mixed with the inert gas, then passed through heated columns to ensure the drug is eventually eluted. Component separation is generally superior to HPLC and mass spectrometers often serve as the detector, but flame ionization detection (FID) and thermal conductivity detection (TCD) are also used.

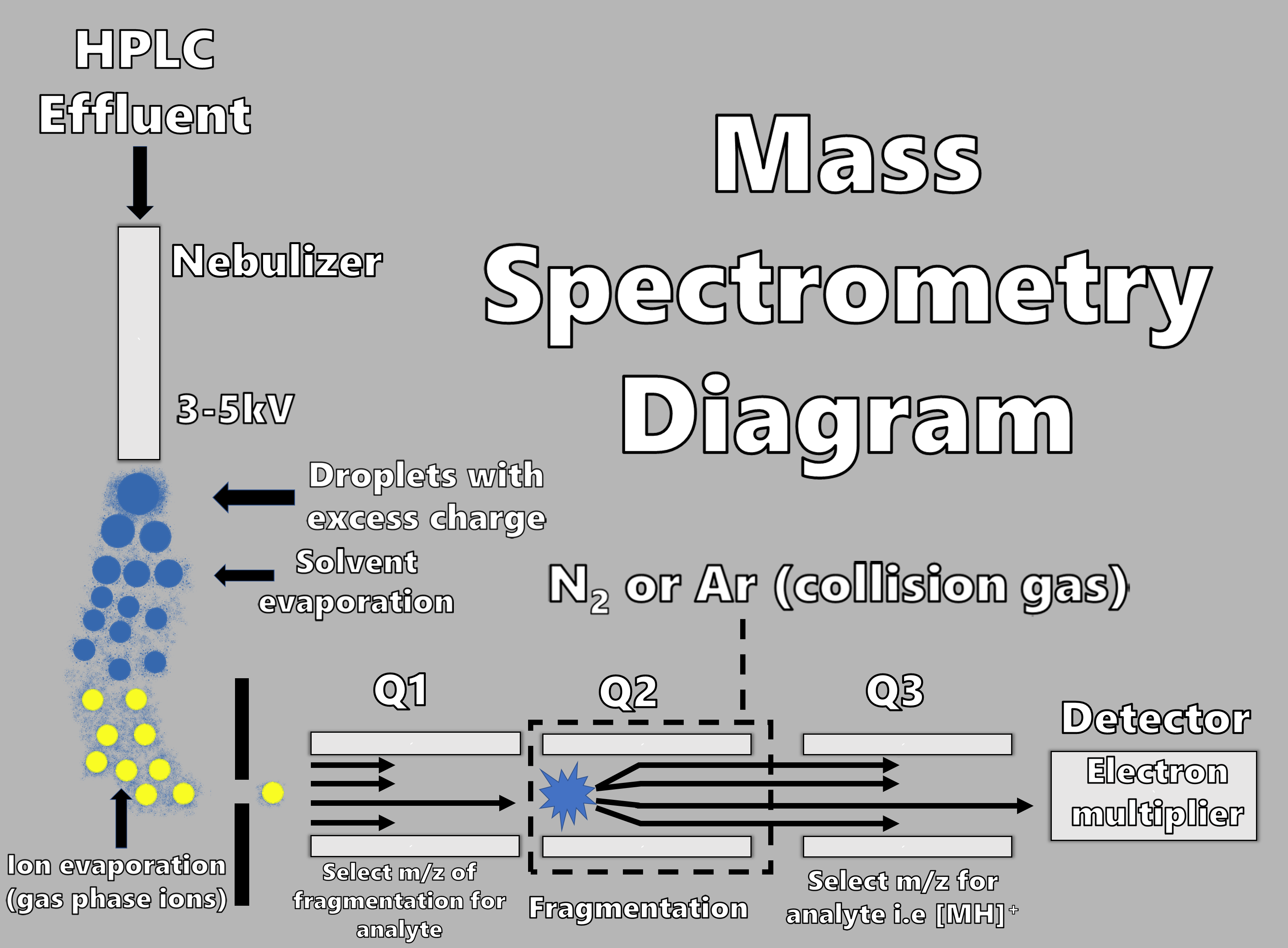
LCMS combines the separation of HPLC with detection by mass spectrometry. Mass spectrometry is used to determine the molecular weight of individually separated components. After the sample has been separated by liquid chromatography, it enters the mass spectrometer, where it is heated to convert it into a vapor which is simultaneously ionized. The ionized vapor is accelerated through a quadrupole (4 equally spaced metal rods) containing a magnetic field and finally into the electron multiplier and detector. Atoms will be deflected by the magnetic field and will then reach the detector depending on their mass. The mass of each component can be scanned and a mass spectrum for each time point can be used to determine the composition of the sample. Greater sensitivity can be achieved by selecting a single mass representative of a specific drug and monitoring just that mass transition. Improved selectivity and an improved signal to noise ratio is achieved by employing a collision cell and monitoring a fragment of the original ion using a third quadrupole, which represents selection ion monitoring or LC-MS/MS. LCMS is commonly used for the analysis of drugs in biological samples to generate pharmacokinetics (PK) and toxicokinetics (TK) data. It is particularly useful for the identification and quantitation of drug metabolites which are not always identified by other techniques.
ITR’s analytical & bioanalytical departments provide support for GLP and non-GLP preclinical and clinical studies by offering dose formulation analysis, analysis of aerosolized drugs and analysis of biological samples to support pharmacokinetics, toxicokinetics and biodistribution in biological samples.
ITR’s dose formulation team supports general toxicology studies for all routes of administration as well as for genetic toxicology studies. Dose formulation analysis and support for inhalation studies is performed using Waters Alliance HPLCs with ultraviolet, fluorescence and evaporative light scattering detectors. These instruments are used to analyze a broad range of drugs in a variety of formulations, including solutions and suspensions. The range of supported drug analyses has recently been expanded with the addition of a new UHPLC, providing increased resolution, sensitivity and speed of analysis.
ITR’s bioanalysis team works from a 96-well plate platform. Routinely used matrices include serum, plasma, whole blood, urine, cerebrospinal fluid, tissue extracts and aqueous/vitreous humor. Extracted biological samples are prepared by protein precipitation, liquid-liquid or solid phase extraction and are then injected into one of our three identical Sciex 4000 LC-MS/MS systems for PK/TK studies. Methods are designed for analyzing eventual clinical samples as well, to ensure continued support as the sponsor transitions from preclinical to clinical research.
We have recently furbished a new bioanalysis LC-MS/MS suite and are in the process of installing a new Sciex 6500+ instrument. This new equipment provides ITR with increased sensitivity, speed and performance, enabling analysis for increasingly smaller sample sizes and a wider category of drugs including proteins and peptides. The new LCMS/MS suite will also have space for an additional three Sciex 6500+ machines to be purchased in the future.

The development of analytical and bioanalytical technologies and their application to the pharmaceutical industry have provided powerful tools to better characterize the safety profiles of new drugs. As the nature and potency of pharmaceuticals change, increasingly sensitive machines have been developed in order to keep up the pace. ITR is also continually upgrading and developing services to match the needs of our clients.