
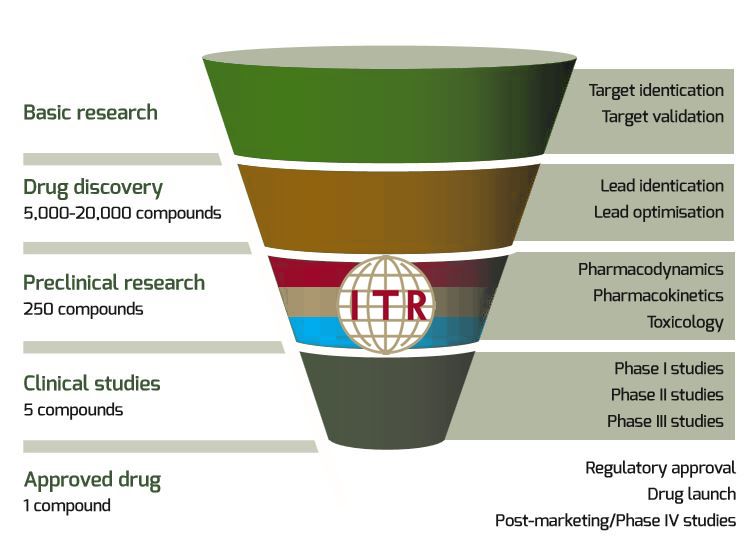
The process of taking a new drug from the earliest stages of development, to the market will require approximately 12 years and an estimated cost of 1 billion dollars. Although the most expensive phase of development are clinical trials, particularly phase 3, the eventual success of these trials requires a strong foundation of effective preclinical research.
The majority of drugs that successfully pass the preclinical stage will eventually fail in clinical trials, leading to major financial losses as well as lost research time that could have been better spent on more promising drug candidates.
The top three cited reasons for these failures are lack of financial support, failures to demonstrate efficacy in humans, and safety concerns.
In this newsletter, we will describe safety research & efficacy research in a preclinical context, we will discuss common problems with preclinical efficacy research and we will present ITRs preclinical services.
What exactly is a safe drug? Safety will depend on a number of factors. What is the drug intended to treat? What is the target population? What are the intended conditions of use for the drug? The answers to these questions will differ from drug to drug and will inform what constitutes a safe drug.
When treating life-threatening diseases, the concept of a safe drug will be different from treatments designed for a headache or the common cold. Hair loss and severe nausea would be unacceptable side effects for cold medicine, but are expected and tolerated with many cancer treatments, this is due to the differences in acceptable risk. A drug may be determined to be safe for adults, but not for children or the elderly. Drug interactions can also compromise safety when dealing with comorbid conditions and polypharmacy.
Preclinical safety research is required by all regulatory bodies in order to minimize the risk of novel drug candidates to humans during clinical trials.
The purpose of preclinical safety research is to predict risk, once risk has been predicted in animals, the next step would be to assess the safety and efficacy of the drug in humans.

While safety research is intended to evaluate the level of risk associated with a drug, preclinical efficacy is focused on predicting the benefits of the drug.
Preclinical efficacy research aims to establish whether the drug will function to treat the target disease or illness in the intended population within a specified condition of use. Unlike with preclinical safety research, efficacy studies are not required by regulatory bodies in the preclinical phase and there are no clear guidelines for preclinical efficacy research.
Efficacy research is sometimes performed in the field of oncology with xenografting and with some specific diseases that can be induced or found in certain animal species, such as type 1 diabetes in the nude rat. Results from preclinical efficacy studies can help provide stronger data to progress the candidate to clinical trials.
Preclinical efficacy research is performed infrequently. The lack of regulatory guidance and expensive nature of drug development in general serve as disincentives, but the challenges extend beyond lack of guidance and financial constraints.

Beyond financial constraints, efficacy research is often omitted due to lack of evidence for supporting drug development beyond standard safety testing. Efficacy results often fail to translate to clinical research.
In order to test efficacy in animals, the disease state must be created in animal models and cross-reactivity with the drug must be demonstrated, which reduces the number of species available for testing. In animal models, the disease state is induced in a variety of ways, but these conditions will often result in different disease onset and progression compared to standard human disease conditions.
The rigorously controlled setting of the laboratory also cannot account for the diversity of human populations in terms of age, multimorbidity, and polypharmacy.
In controlled laboratory settings, the disease is often treated earlier than is standard in human populations. Human disease is often only treated once symptoms begin to manifest, which can be long after the disease could be detected and diagnosed. This contrasts the research setting where the disease is induced and can be treated earlier.
Without controlling for all these factors, external validity of results is compromised, the effectiveness of the drug may then be overestimated or underestimated. So long as we can predict the level of risk a new drug poses to humans during clinical trials, efficacy is better studied in humans.
ITR specializes in preclinical safety research, after 30 successful years of operation, our expert staff can offer a diverse range of toxicology services.
Our core toxicology services include drug administration via the oral, inhalation, intravenous (bolus and continuous infusion), subcutaneous, intramuscular, dermal and ocular routes, as well as immunotoxicology, genetic toxicology and safety pharmacology studies.
With over 6000 preclinical studies successfully performed, ITR has extensive experience and offers a wide range safety studies. We can test on small & large molecules in both small and large animals at a single site. We can test across all phases of preclinical development including IND enabling studies as well as NDA, ranging from acute to carcinogenicity studies.

Preclinical research is a mandatory step to producing safe and effective drugs. Although efficacy is sometimes tested preclinically, the lack of regulatory guidance and unreliable results call its value into question. The questionable value of the research also brings up ethical concerns, minimizing unnecessary use of animals is a driving factor in what research is performed.
ITR is committed to providing the highest quality preclinical safety research, direct communication with our scientific leadership helps our clients smoothly advance their drug candidates through to the clinic.
