
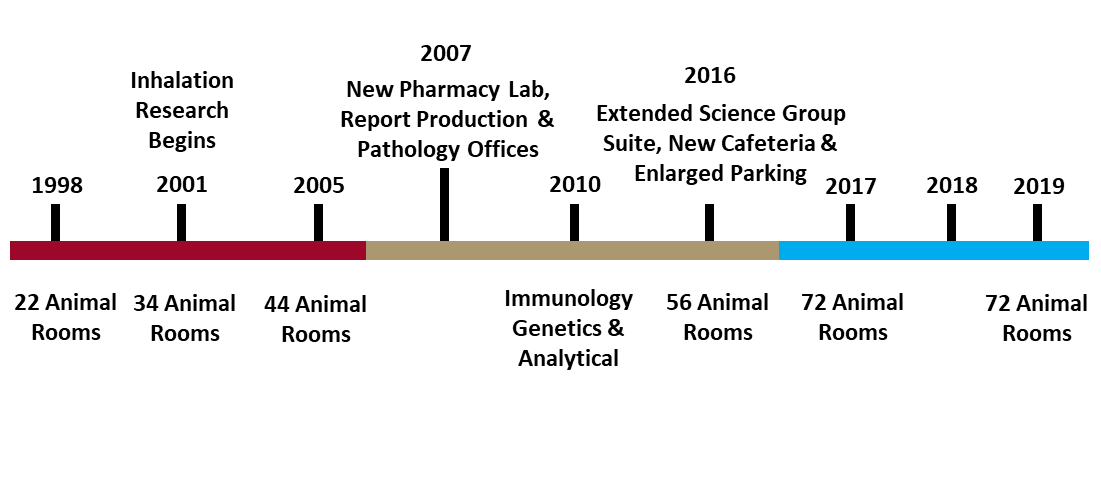
In 2019, ITR Laboratories is celebrating 30 years of consecutive organic growth. In this newsletter, we will recount the history of ITRs development. First, we will begin with a timeline overview of our development from ground-breaking day in July 1989 up until today. Then we will look at each phase of ITRs development in more detail. Finally, we will discuss modern developments at ITR.


ITR was first established as a specialized infusion CRO in 1989, our doors opened for business in May of 1990. Beginning with only 10 animal rooms, and roughly 50 staff, our work was limited in scale, the toxicology staff was responsible for pharmacy in addition to general toxicology services. Successful work for 8 years enabled ITR to expand in 1998 to include another 12 animal rooms, increasing our total to 22 rooms. In 2001, ITR widened the scope of our research capabilities including inhalation research, increasing our number of animal rooms by another 12, with 6 designed for inhalation exposure studies, animal rooms now totaled 34. In 2005, another 10 rooms were added. 2007 saw the construction of expanded office areas for Report Production & Pathology, as well as a Pharmacy Laboratory.
In 2010, ITR again expanded its services to include Immunology & Genetic Toxicology. Now with capabilities in Infusion, Inhalation, Immunology & Genetic Toxicology, ITR staff was growing rapidly, which necessitated the expansion of our Science Group Suite, Cafeteria and parking areas in 2016. An additional 12 animal rooms, were also added in 2016, bringing the total up to 56. In 2017, yet another 16 animal rooms were added, along with another expansion to the parking area and access gates around the facility. In 2018, a second Tissue Culture Lab was constructed for the Immunology department and increasing demand for Inhalation research resulted in the conversion of 4 rooms to support Inhalation exposure studies. Over 30 years, ITR has successfully managed consistent organic growth and is now operating with over 400 staff and 72 animal rooms, next we will look more in depth at the evolution of our services & technology.
ITR was founded as a specialized Infusion CRO. On opening day in May of 1990, we were operating with a staff of roughly 50. We had a well-equipped surgical suite, a large inventory of syringe & peristaltic infusion pumps as well as jacket & tether systems with specially designed cages. Formulations were administered via an exteriorized catheter implanted in a femoral vein, disposable catheter via a peripheral vein or by Vascular Access Port, dosing ranged from minutes to 24 hours a day for up to 9 months.
Along with Infusion, Dermal research at ITR was among our earliest services, dating as far back as 1996. Capable of performing topical administration with gels, creams, patches emulsions and sprays for single dose or chronic studies with cynomolgus monkeys, rabbits, rodents, mini-pigs and dogs.
As part of our general toxicology services, ITR also performed toxicity research with orally administered test items as well as ocular irritation & tolerability studies with drops as early as 1999. As ITR developed, our ocular capabilities expanded to include topical instillation performed by our technical staff, as well as intraocular injections and ocular implants, performed by board certified ophthalmologists.
Today, ITR is one of the world leaders in non-clinical infusion technology, having performed over 1000 infusion studies, we have also invented new technology to enable dog exercise on continuous infusion studies: The Needleless Injection Site Swabbable Connector (NISSC) (Fig.1), which enables dogs to be safely disconnected from the infusion pumps temporarily for exercise without risk of infection or catheter displacement.

In 2001, ITR constructed 6 new animal rooms and fitted them with the necessary equipment to perform inhalation research. ITRs inhalation research began with two rooms for small animals fitted with European custom designed cabinet systems (Fig.2) as well as four exposure rooms for large animals.
Using Dry Powder Generators, Metered Dose Inhalers and Nebulizers, we were capable of supporting all forms of aerosol suspensions, gas, liquid and powder. Our modular Flow-Past system for small animals supported 16 animals per stage with multiple stages. The large animal exposure system (Fig.3) enables group dosing of 10 animals per group. On-site analytical chemistry capabilities validated and characterized exposure aerosols prior to dosing for aerosol concentration and particle distribution. With increasing demand for inhalation services, ITR expanded the inhalation area, increasing the total number of inhalation rooms to 20. By 2019, inhalation makes up 40% of ITRs revenue.
ITR has also been named as the highest performing CRO for Inhalation/respiratory toxicology, more information can be found on our website: https://www.itrlab.com/wp-content/uploads/2018/11/Contract-Pharma-CRO-analysis-news.pdf


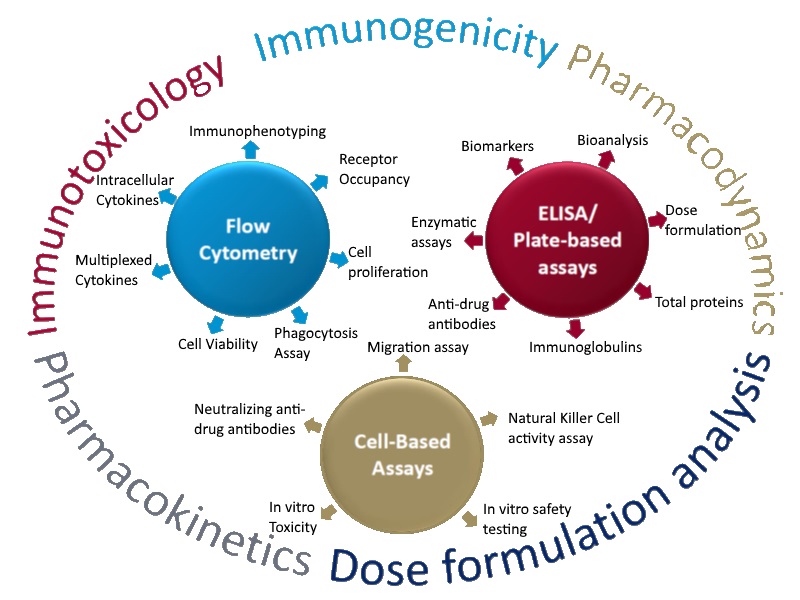
In 2010, ITR established an immunology department in response to increased industry demand for biomarker assays, large molecule bioanalysis, immunogenicity testing and cell-based assays. Beginning with the purchase of a plate reader and washer, ITRs immunology department was developed from the ground up. After including Flow Cytometers to the list of available equipment, the demand for immunology services kept the department busy between 2011 and 2012, working mostly with ELISA / Plate-based assays.
Genetic Toxicology was also included in 2012 to suit clients needs, as small molecule development requires Genetic Toxicity testing. Two genetic toxicologists were hired. Having now grown to 15 staff, the genetic tox team worked closely with immunology, cross training to help spread the increasing workload.
From 2011 – 2016, Immunology & Genetic Tox continued to operate, adding Receptor Occupancy to the list of available services. From 2016 – 2019 the department doubled its staff from 15 to 30. Over the last 8 years, Immunology & genetic tox has performed nearly 300 preclinical studies with large molecules and has developed & validated 75 test item specific methods to support biotechnology-derived drugs including recombinant proteins, fusion proteins, peptides/hormones, monoclonal antibodies and enzymes. 2018 saw the opening of a second tissue culture lab as a result of record breaking revenues and 2019 projections show no signs of slowing down.

Today, ITR is working to develop more techniques for testing with juvenile & neonatal animals in response to increasing development of pediatric medicines. Both humans and animals in early development phases will respond differently to medication. In an effort to increase the availability of medicines to newborns and young children, testing neonatal & juvenile animals is required and ITR is meeting this demand.
Juvenile testing however has a dearth of available data to establish proper baselines, as such, ITR has collected data from nearly 400 Sprague Dawley rat pups at 4, 7 and 21 days of age to compare against available datasets for adult rats. ITR has also developed methods for intravenous and oral dosing of neonates from one day after birth for as long as required. Using a magnifying or dissecting microscope, intravenous dosing in neonates is performed via the temporal or facial vein, oral gavage is performed using a conventional plastic mouse gavage cannula. Up to 9999 neonates can be separately identified and tagged with our dot tattoo digital identification system. Developments in pediatric medicines are now leading towards development of medications for prematurely born children, as a result, ITR is now looking at developing methods for pre-natal testing as well.
ITR has tested vaccine products since our early history. In recent years, we have adopted newer vaccine administration technology that does not require the standard intramuscular or subcutaneous needle & syringe administration. Consistent with our commitment to the 3R’s, newer intra/transdermal administration methods are virtually painless. Utilizing the 3M micro-needle technology™, Debiotech™ hollow micro-needles and NanoPass MicroPyramid™ systems, we not only take advantage of the skins antigen-presenting cells to produce a stronger immune response, we reduce the pain that results from standard intramuscular administration.
In January of 2019, we completed the validation of a new GC-MS equipment to add to our list of available analytics equipment. Along with our LC-MS machine, we are capable of analyzing both non-volatile and volatile compounds, granting more flexibility for sample analysis. Going forward, ITR is looking into the world of Regenerative Medicine and Medical Devices.

Over 30 years, ITR has grown organically from a small facility with capabilities limited to general toxicology and infusion, to a world leader in inhalation research and a top performer in non-clinical infusion studies. Our Immunology & Genetic Toxicology departments were established from the ground-up and are showing record breaking revenues year over year.
Developing new methodologies as in our work with juvenile and neonatal rats, and pioneering new technologies such as the Needleless Injection Site Swabbable Connector, ITR will continue to wade into new territory to maintain our flexible approach to new industry developments. However the world of medical science evolves, ITRs evolution will swiftly follow.