
Lung disease remains one of the leading causes of death globally. The World Health Organization classifies Asthma, Chronic Obstructive Pulmonary Disease (COPD), Acute Lower Respiratory Tract Infections, Tuberculosis and Lung Cancer as The Big Five in lung disease. These five lung diseases are the most common causes of severe illness and death worldwide. In 2017, an estimated 3 million deaths resulted from COPD alone with an estimated 200 million people suffering from the condition. Lung cancer still results in even more deaths, with an estimated 9.6 million deaths in 2018 and the number of people suffering from asthma worldwide continues to rise.
Although ITR opened its doors in 1989 as a specialized infusion CRO, it soon branched off to inhalation toxicology. Today, ITR is a global leader in respiratory toxicology research. With nearly 500 Inhalation studies conducted to date, we have progressed developments towards treatment for serious lung disease such as COPD, Asthma, Pulmonary Fibrosis and Cystic Fibrosis.
Although ITR opened its doors in 1989 as a specialized infusion CRO, it soon branched off to inhalation toxicology. Today, ITR is a global leader in respiratory toxicology research. With nearly 500 Inhalation studies conducted to date, we have progressed developments towards treatment for serious lung disease such as COPD, Asthma, Pulmonary Fibrosis and Cystic Fibrosis.
With 20 available exposure rooms for inhalation research, we offer of a wide variety of aerosol generation methods, aerosol monitoring and analytical chemistry support, ITR is prepared to perform a wide variety of inhalation research.
In this newsletter we will discuss ITRs infrastructure, technology, experience and expertise with inhalation research.

ITR is a purpose built GLP facility for pharmaceutical development. After opening its doors in 1990 and performing general toxicology research for 10 years, inhalation research services were added to our capabilities. Now equipped with 8 exposure rooms for small animal exposure and 12 exposure rooms for large animal exposure, the rooms are fashioned with ventilation systems designed to limit cross-contamination possibilities and to minimize exposure of concurrent control animals to test materials. Separate treatment and control rooms are employed for large animals to eliminate the possibility of cross contamination. The inhalation suite supports the full spectrum of inhalation regulatory studies.
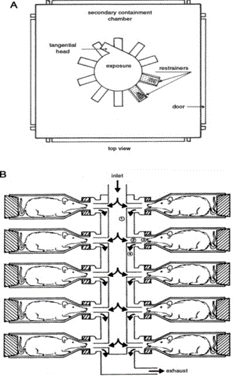
Our exposure systems are based on Flow-Past technology, aerosol flows past each port and exhaled air is immediately exhausted. This system allows for all animals in the chamber to be exposed to the same concentration of aerosol.
Our system for small animals (Fig. 1) enables dosing of 16 small animals per stage with the possibility of using several stages in an exposure system. The nose-only system allows us to achieve the required dose while minimizing the amount of test article used. In addition, it eliminates potential eye irritation. Our chemical and temperature resistant polycarbonate restraint tubes for rodents are available in a range of sizes to accommodate multiple ages and rodent species. The rodent restraints are tail-free and designed to keep rats comfortable for longer exposures.
Large animal exposure (Fig. 2) is accomplished with face-masks using low volume chambers. Our 12 large exposure rooms support group dosing with up to 10 large animals simultaneously. Custom designed platforms are used for dogs and custom chair restraints for primates to ensure the animals are comfortable for extended exposures or multiple exposures.

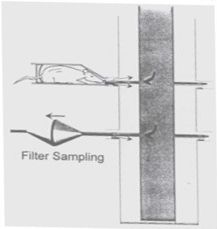
ITR is capable of generating aerosols using a variety of methods. Rotating Brush Generators (RBG) are used for dry powder aerosols. Fine control of dry powder aerosols is achieved using a combination of rotation speed, brush type and canister size. An in-line micronizer can further optimize dry powder aerosols by controlling particle size and significantly reducing test item requirements.
We can also use liquid or suspension aerosols by integrating many types of nebulizers into the exposure systems, including Airjet, electronic/mesh nebulizers and atomizers. Infusion pumps are used to maintain constant nebulizer volumes during aerosol generation.


ITR works in tandem with our Sponsors to mutually understand, characterize, qualify and quantify aerosols prior to commencing inhalation toxicology trials. Our dedicated specialists work towards building a strong foundation for aerosol development, ensuring the atmosphere is respirable, reproducible, homogenous, efficient, and on target. We employ isokinetic sampling philosophy in a variety of sampling mediums and logistics to support monitoring of the aerosols.
Our Analytical Chemistry labs, equipped with LC-MS, GC-MS, HPLC and UPLC equipment, provide further support and can facilitate transition from method development towards completion of validation process to ensure GLP-readiness prior to commencing inhalation toxicology preclinical trials.
We assess assay specificity, accuracy & precision, active ingredient content recovery and stability of aerosol concentration and particle size samples.
Pharmaceutical research is expensive and time consuming, for those in the business of developing new drugs, it is paramount to have a carefully planned approach. Good market research is essential to reducing the potential risk involved with developing new drugs. In June of 2018, an independent senior business consultant evaluated the preclinical toxicology CRO landscape using company websites, downloadable brochures, financial reports, Linkedin pages, press releases and IP databases.
Two dimensions were used for ranking:
Commercial Capabilities defined as: the solidity and ability in defining and articulating the current and future strategy.
Technological Capabilities defined as: the delivered services and market adoption.
44 CROs were identified and scored, only 26 received a score greater than 5. For this phase of selection, ITR was identified as #4 in terms of commercial score and within the top 5 in terms of technical score.
The remaining 26 companies were segmented into four separate groups, Biobanking/Omics/Bioinformatics, Crowdsourcing Platforms/Science Verification, General Toxicology and Inhalation/Respiratory Toxicology. A set of more detailed criteria was defined, including but not limited to: Company disposal of products, services, skills, resources & technology, quality of service, Innovation, Pricing Model, Customer focus, etc. Each of these criteria was scored from 1-10 and each criteria was given a weight from 10%-30%, finally each company was given an aggregate score and they were graphed into their four separate groups.
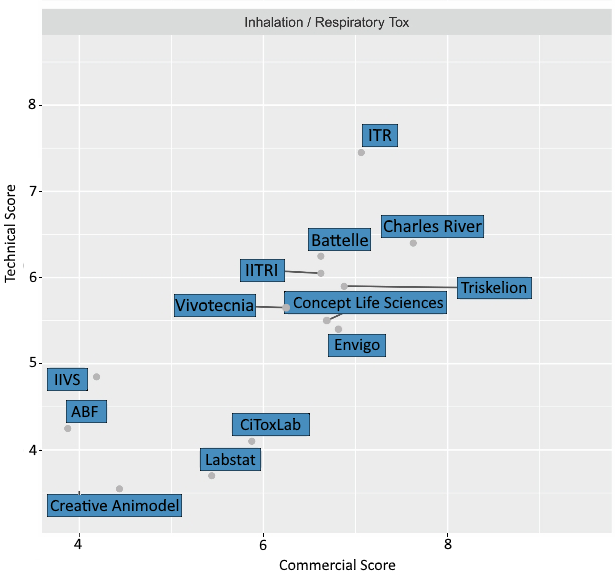
ITR was sorted into the inhalation segment. In terms of Technical score, ITR scored highest with a 7.5, one solid point above the cluster of companies that scored between 5.5 and 6.5. In terms of Commercial score, ITR scored second highest with 7. ITRs position at the top right hand side of the graph on the next page indicates a leadership position in Inhalation/Respiratory Toxicology.
The graph below displays how ITR was positioned in relation to the other CROs who were grouped together in the inhalation segment of the analysis.
ITR was positioned as #1 in terms of Technical Score and #2 in terms of Commercial Score.
After 18 years of respiratory toxicology research and over 500 successfully completed studies, ITR has been propelled to a globally competitive position in inhalation research, and today inhalation research comprises 40% of ITRs revenue. With plans to increase our available exposure rooms to include several new rooms in 2019, ITR will continue to invest in our inhalation capabilities to maintain the best quality services.
