
When predicting safety profiles of developing drugs, researchers have relied on the use of animal models, given that biological processes that occur in animals are often also seen in humans.
Today, biomedical research places strong emphasis on the health and comfort of research animals, under the guidance of the 3Rs: Replacement, Reduction and Refinement. In order to continue improving the ethical landscape of preclinical research while maintaining or improving the quality of scientific data, alternatives to animal research are under continual development. One such alternative is the use of in vitro models.
In this newsletter, we will delve into what in vitro research is, what it can accomplish, and how it relates to the 3Rs. We will also present what ITR can offer in terms of in vitro options and other developments to further reduce, refine and replace animal models.


Standard in vivo research involves administering a test article to an animal, collecting biological samples and performing tests to evaluate the effects of the substance.
In vitro research does not rely on live animal models. Instead, it is performed by isolating components of an organism from its standard biological surroundings and administering the test article into artificial culture media. The term “In vitro” translates to “In glass” in Latin; the use of petri dishes, test tubes and flasks are standard with in vitro research.
In vitro methods have evolved over time. Today, these methods help with reduction and refinement of research in a number of ways; modern cancer research, for example, uses monoclonal antibodies (mAbs) to harness the human immune system to destroy cancer cells. The use of mAbs limits the systemic damage that results from traditional radiation therapy.
The reliance on animals for the production of mAbs has been reduced in modern research through the use of in vitro methods: instead of relying on animals for production, cell lines can now be used instead.
Aside from reagent production, in vitro methods also enable researchers to more quickly identify promising drug candidates with the use of high throughput screening processes. Early efficacy research can be performed in vitro and researchers can also develop a mechanistic understanding of a compound and determine the pathways involved with a variety of toxicological endpoints before in vivo research is conducted.
Overall, in vitro research has provided methods to make the research process more efficient, and has helped reduce the need for the use of animals.

Isolating organic components from the larger biological systems allows for highly controlled, standardized and easily replicable experimental conditions. In vitro tests can be performed quickly and are comparatively inexpensive, requiring smaller amounts of test material and limiting the amount of waste produced. This is what allows for a large library of potential compounds to be screened quickly and efficiently to help narrow down on promising candidates in the early stages of drug development. In vitro tests can also involve use of human cells and tissues to identify potential issues in translating animal data to suit human applications.
While in vitro testing is helpful during early stages of drug development, it cannot serve as a complete replacement of in vivo research. In order to demonstrate the safety of a drug for human use, we need pharmacokinetic and pharmacodynamic data. This requires a full and intact biological system for the drug to interact with. Since in vitro tests are only performed on isolated biological materials, this data is not possible to gather. Reactions between different cells, tissues and organs cannot be studied, nor can the effects caused by chronic exposure.
While progress has been made with artificial organs for testing purposes, they are not yet developed enough to provide sufficient data to make strong predictions of safety in humans.

All regulatory bodies require genetic toxicology screening for new drugs prior to clinical testing. Much of the required genetic toxicology tests can be performed in vitro. However, although there has been great progress with the development of In vitro genotox methods, they are still not yet capable of entirely replacing in vivo methods for a complete safety profile of developing drugs. Mutagenicity may go undetected in vitro, but subsequently found in vivo.
Regulatory bodies recommend a core-test battery of the bacterial mutation assay combined with the in vitro micronucleus assay (CHO-k1 cells). This combination provides information on three types of genetic damage for which data are required: gene mutation, changes to the number of chromosomes (aneuploidy) and their structure (clastogenicity).
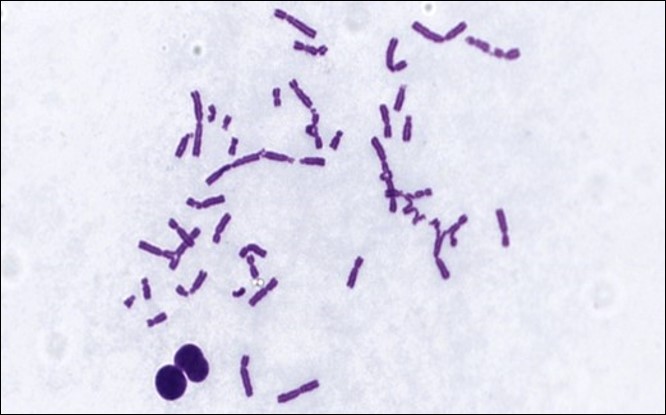
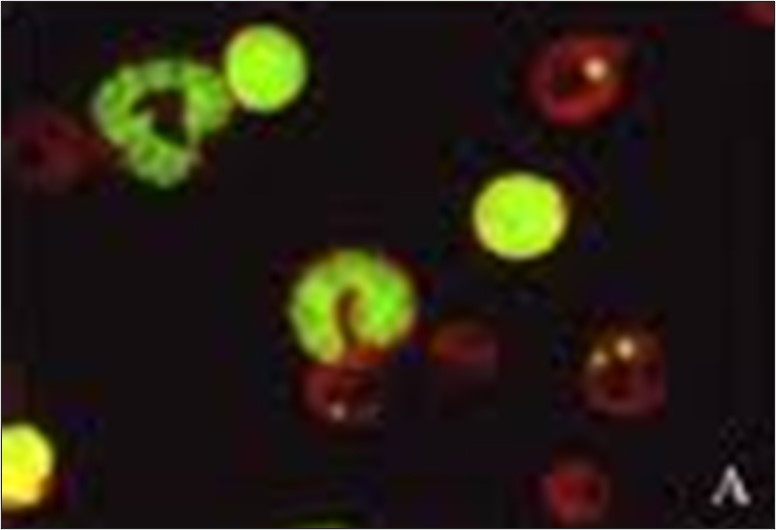
The in vitro chromosome aberration assay, the in vitro micronucleus assay and the mouse lymphoma gene mutation assay (MLA) assays are currently considered appropriate tests for the assessment of the genotoxic potential of a drug candidate (ICH S2-R1). These, along with a variety of other genotoxicity tests, are part of ITR’s portfolio of capabilities.

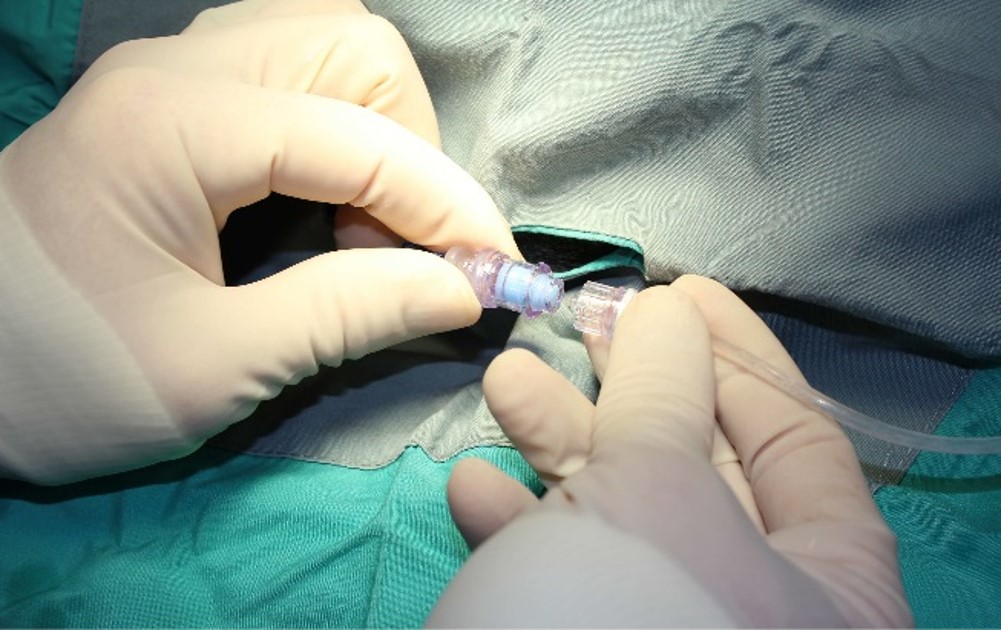
Over the years, ITR has progressively made changes to improve the lives of our research animals. Upgraded housing conditions allowing for more space, more developed enrichment programs and innovative technology like the Needleless Injection Site Swabbable Connector which allows dogs to exercise during continuous infusion studies have been significant developments.
Today, our Meso Scale Discovery (MSD) Quickplex Plate Reader has allowed us to greatly refine plate based assays. A standard ELISA requires 200 microliters of serum (approximately 450 μL of blood) and allows for the measurement of only 1 cytokine at a time. However, the MSD Plate Reader allows for the measurement of up to 10 cytokines. This multiplex technology also reduces the amount of required blood by a factor of 80 (2 mL vs 25 uL) while providing greater quality results by reducing intra and inter-assay variabilities.
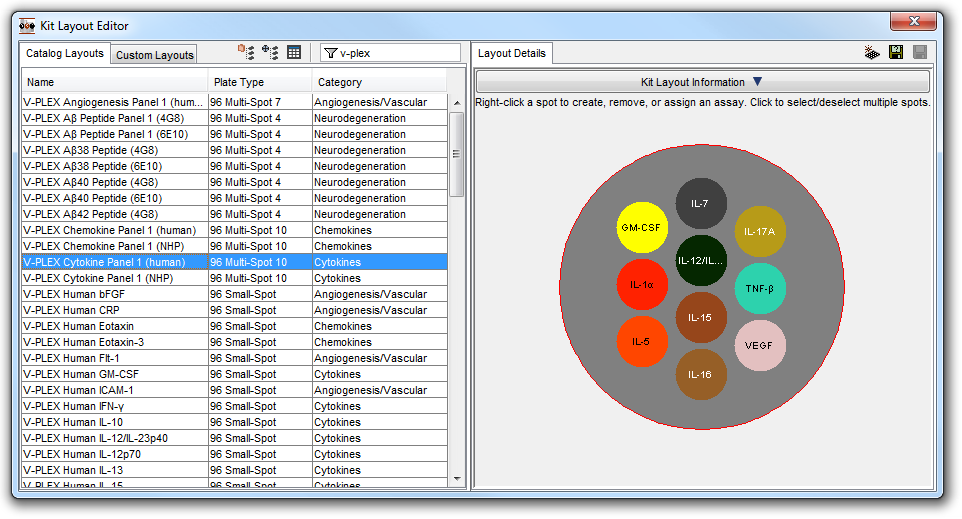

While in vitro methods have made considerable developments in recent years, the complete replacement of in vivo testing is still many years away from becoming a realistic approach to safety testing. ITR is always searching for more ways to reduce, refine or replace animal models. Continual improvements to our procedures and technology have made meaningful differences; for example, with the MSD Quickplex plate reader, ITR can evaluate a greater number of preclinical biomarkers while drawing significantly less volumes of blood, putting less stress on the animals.
ITR will always remain committed to continued Replacement, Reduction and Refinement to help improve the lives of research animals.
